Cell-autonomous size homeostasis in mammals
How are cell growth and cell cycle progression coupled?
Why study cell size?
Size is one of the most fundamental parameters of a cell. Yet, we do not understand what sets a cell’s size or how they maintain that size over time.
This mechanism is especially important in cell types in our bodies that are constantly proliferating – that is, our stem cells. Both growth and division can change cell size, and must be coordinated to keep cell size stability.
And why does cell size stability matter? For a single cell, its size determines the concentration of its genome, which limits how much machinery cells have access to to grow and perform their functions. In multicellular tissues, cell size dictates how tissues are organized, how cells move, how cells touch each other, and other physical forces driving cell behaviors.
Despite this, cell size has not yet been systematically studied in multicellular tissues.
What we knew (about yeast) and didn’t know (about mammals)
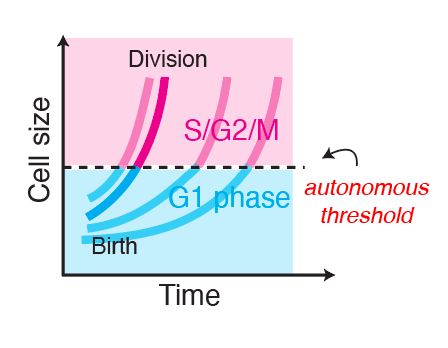
Budding yeast cells coordinate their G1-to-S cell cycle transition to cell size. This way, two cells that were born at different sizes end up more similar in size as they enter S phase.
However, despite more than a decade of research in cell culture models, it was unclear whether mammalian cells coupled their cell cycle progression to their cell growth in quite the same way. Different cell lines seemed to have different cell size behaviors, and it was argued that for metazoan cells, the coordination between cell growth and cell cycle mainly occurred at the signaling level, and was not autonomous to the cell.
We knew next to nothing about cells in vivo, however. Therefore, I developed an experimental system experimental system in which I can study cell size homeostasis in an in vivo animal, using intravital imaging to directly observe how single cells grow and divide in the living mouse skin, in 4D.
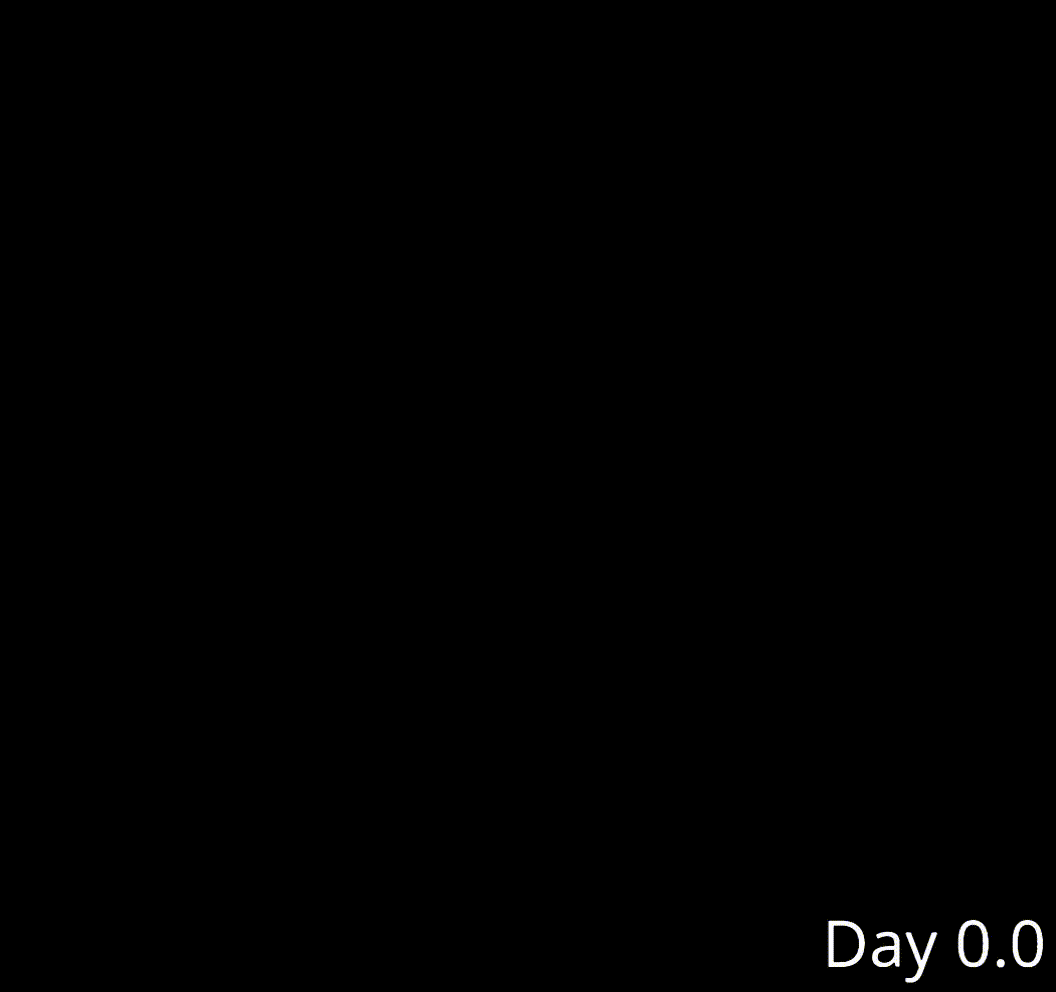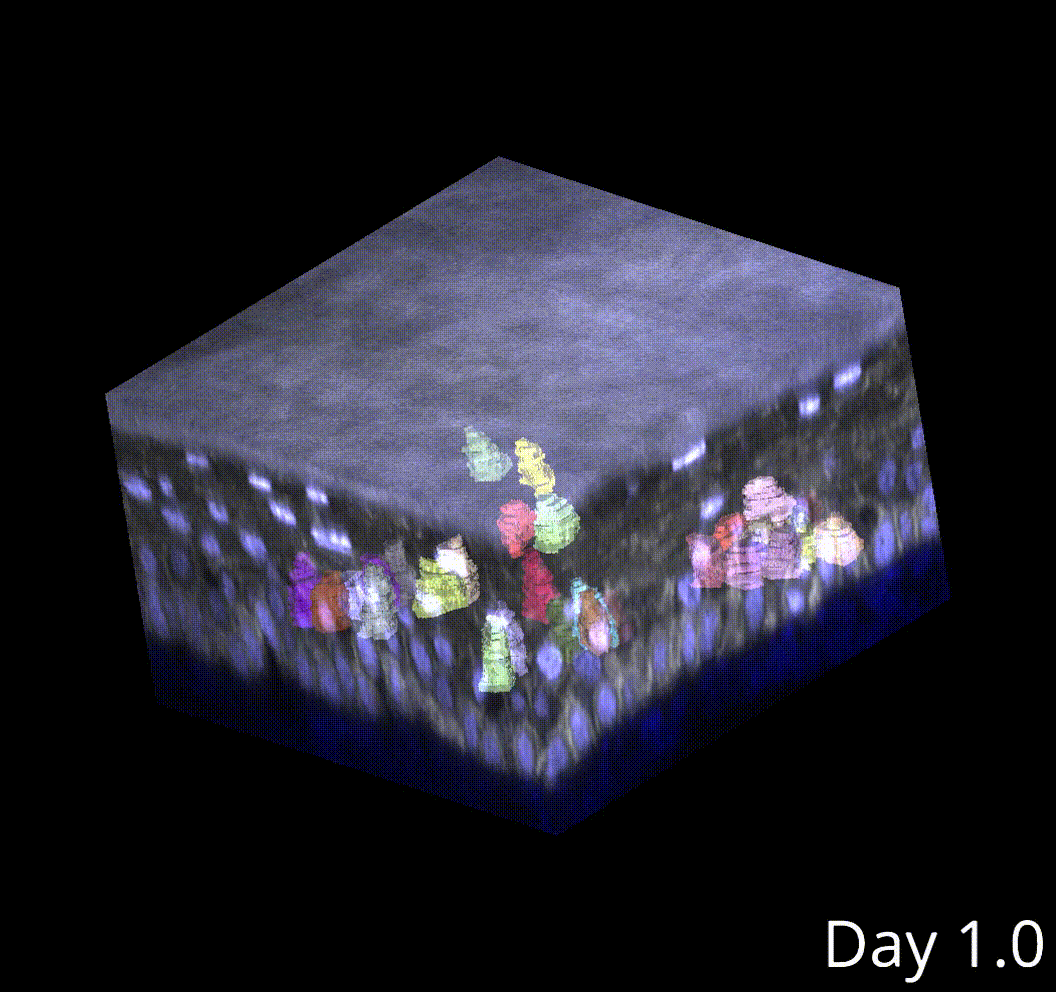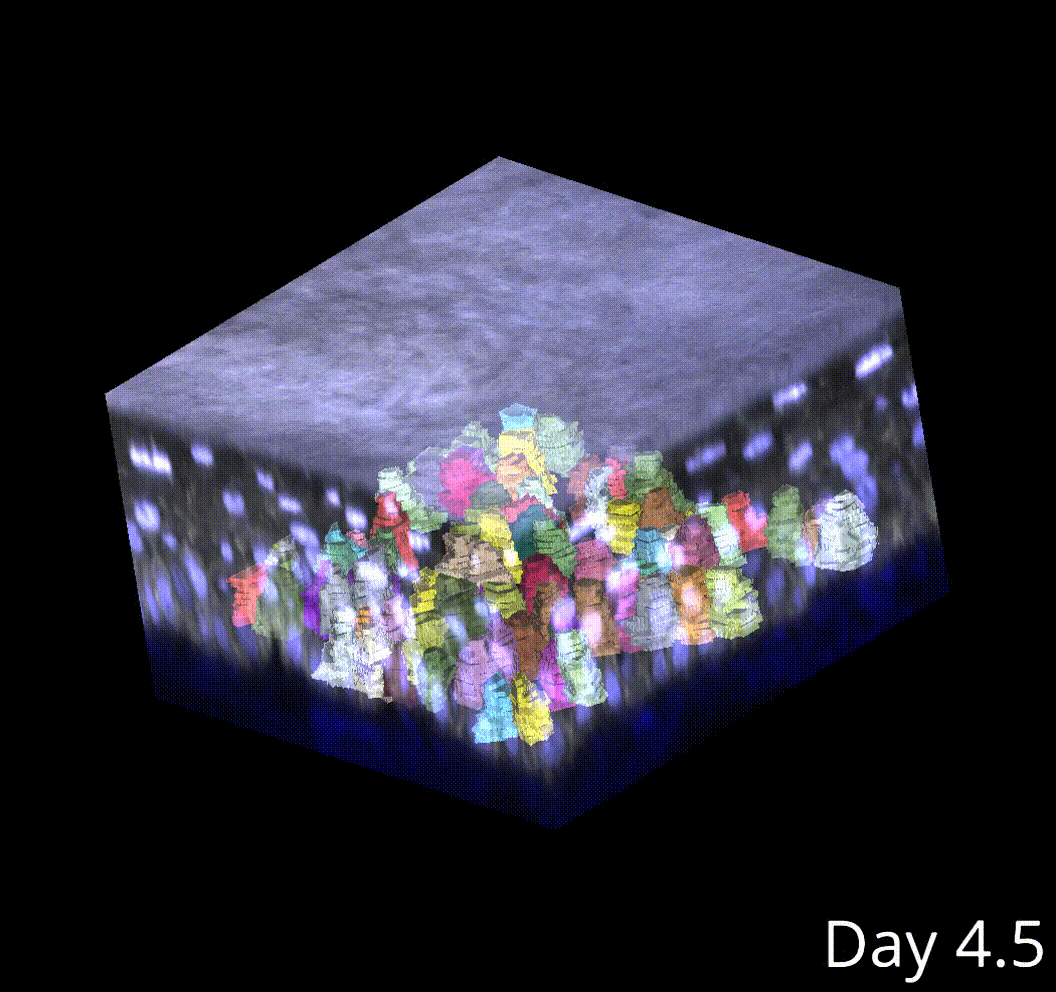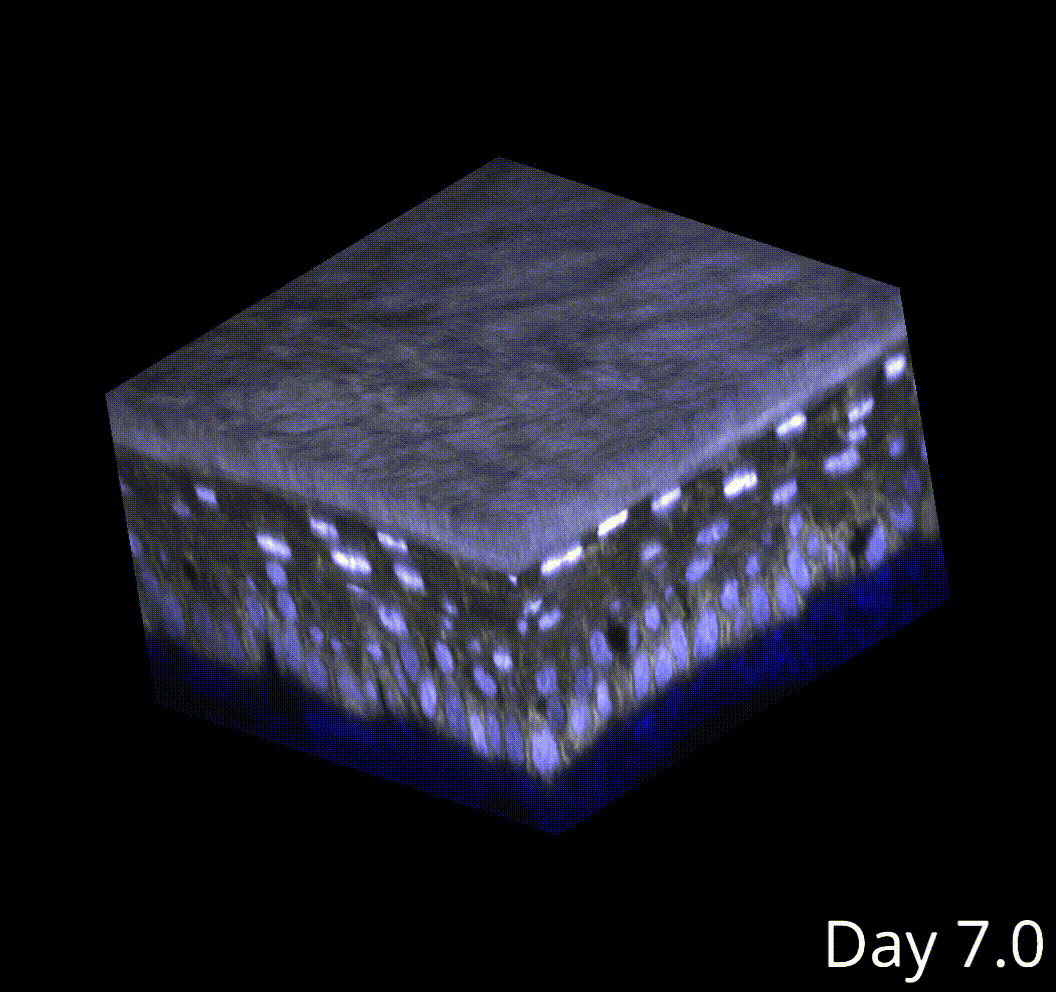
The G1/S transition in vivo is sensitive to cell size


This coordination between cell growth and the G1/S progression is very similar to what was observed in budding yeast, and contrary to the majority of cell culture models.
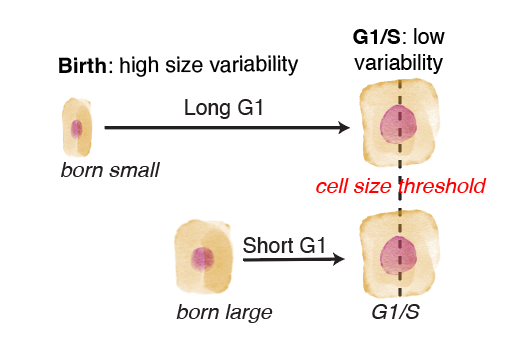
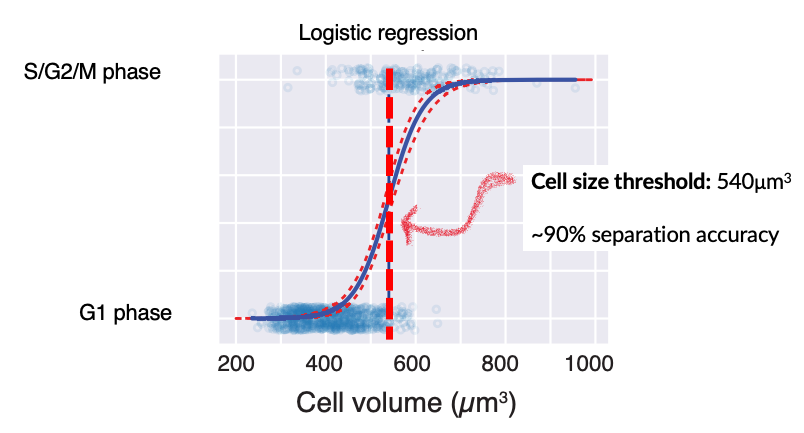
The G1/S transition happens at an autonomously-encoded cell size threshold
Clearly, cell size is influencing whether skin stem cells in vivo enter S phase or not at any given time. However, it was unclear how much influence cell size has on this key decision, compared to the slew of other environmental signals and changes these stem cells experience every day.
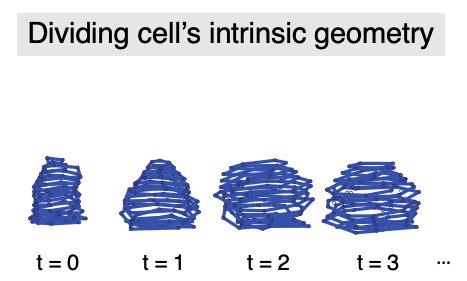
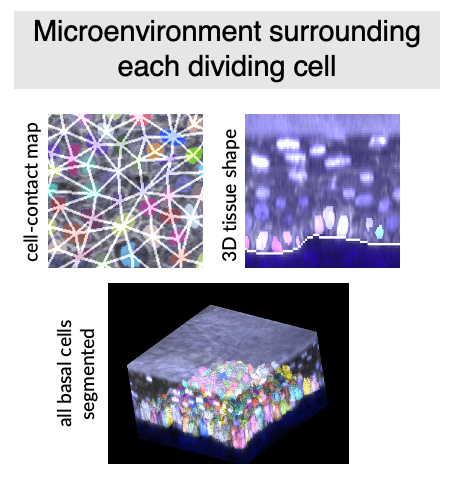
Using quantitative image analysis, I analyzed how the dividing cell’s morphology as well as the tissue microenvironment surrounding it changed over time. Then, using this rich set of information of cell and microenvrionment morphometrics, I built statistical models to isolate which feature can predict whether cells will enter the G1/S transition. (Xie et al., 2024)
Perturbing the microenvironment using laser cell ablations did not change this cell size threshold in neighboring cells, despite driving faster growth rates in those neighbors. Therefore, this threshold is autonomous to the dividing cell.
G1/S cell size threshold accounts for ~65% of stem cell cycle heterogeneity
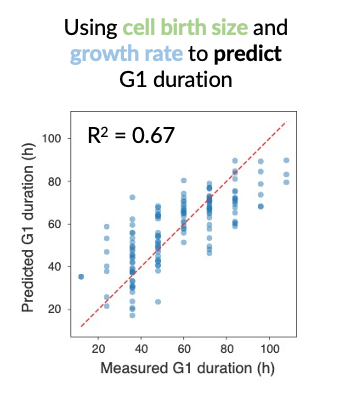
The in vivo skin stem cell cycle is highly heterogeneous. For the mouse hindpaw, it could be as short as 1 day or longer than 1 week. G1 phase is by far the most variable cell cycle phase in vivo, accounting for 96% of the total variation in cell cycle. I showed that a combination of two factors, cell size at birth (how close to the threshold a cell starts at) and cell growth rate (how fast a cell approaches the threshold), can accurately predict the total duration of G1.
Conclusion
The high heterogeneity cell cycles of adult stem cells in vivo is due mainly to cell size homeostasis mechanisms. That is to say, the cell cycle progression in vivo is stringently coupled to cell size, and variation in cell cycle length serves to maintain uniformity in cell size. Why cell size uniformity needs to be actively maintained, however, is still a mystery.