Coordination of actin-myosin contractions
How does a thousand cells coherently generate force to bring about tissue-level folding?
Pulsatile actin-myosin contractions fold tissues
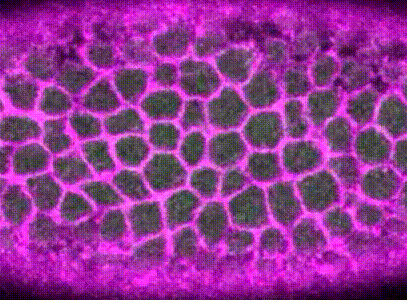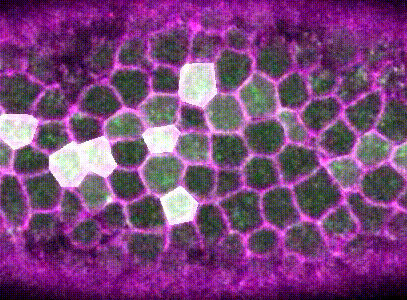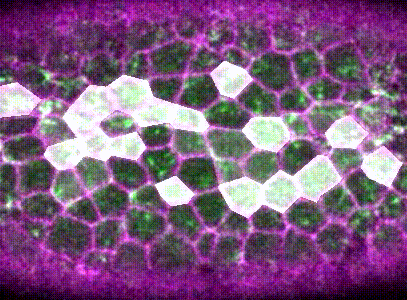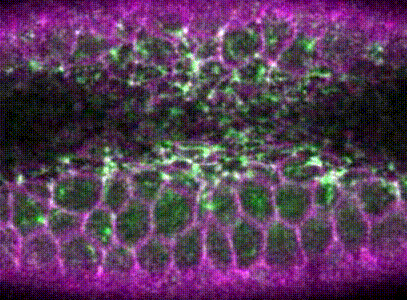
During Drosophila gastrulation, around 1,200 mesoderm cells collectively contract to form a fold in the tissue. The force necessary for this morphogenesis is (partly) generated by actin-myosin contractions at the apical surfaces of the mesoderm cells.
These actin-myosin contractions are pulsatile, meaning they occur in discrete steps, driven by the phosphorylation dynamics of myosin; each pulse of myosin activation leads to a discrete constriction of the cell apical surface.
How are discrete pulses spatiotemporally coordinated?
I became interested in how a large, mechanically coupled sheet of epithelial cells coordinate these discrete contraction events in a way that eventually propagates tissue tension across a millimeter in length.
I built computational methods to extract when and where these contractions occurred in the embryo, using a combination of image analysis and time-series analysis. (Xie & Martin, 2015)
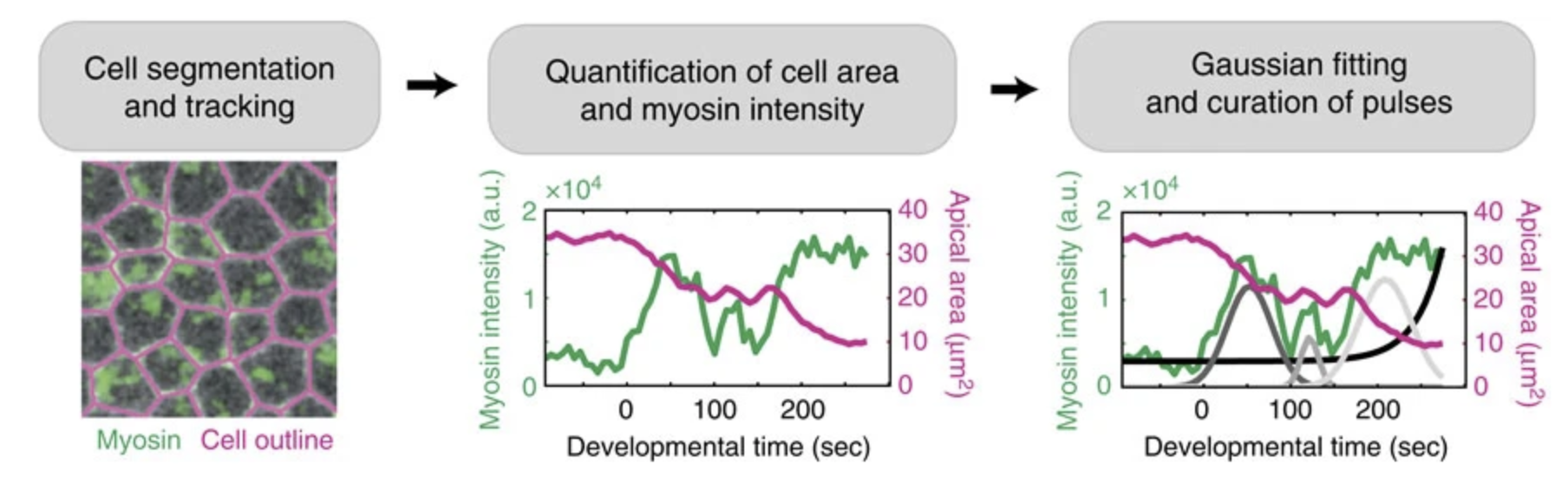
Then, using machine learning, Monte Carlo methods, and spatial statistical analysis, I found that neighboring pulses are coordinated within the epithelium. Cells that are next to contractions are more likely to mount a contraction of their own. Contractions that are next to other contractions are also more likely to be irreversible.
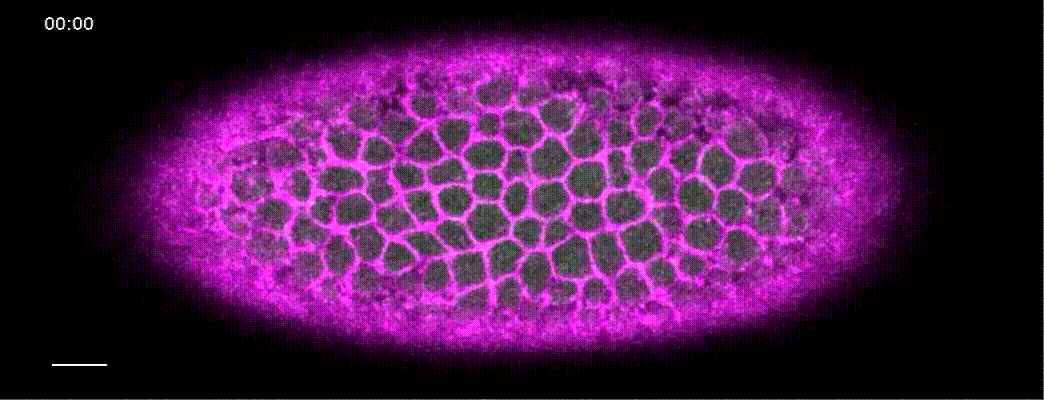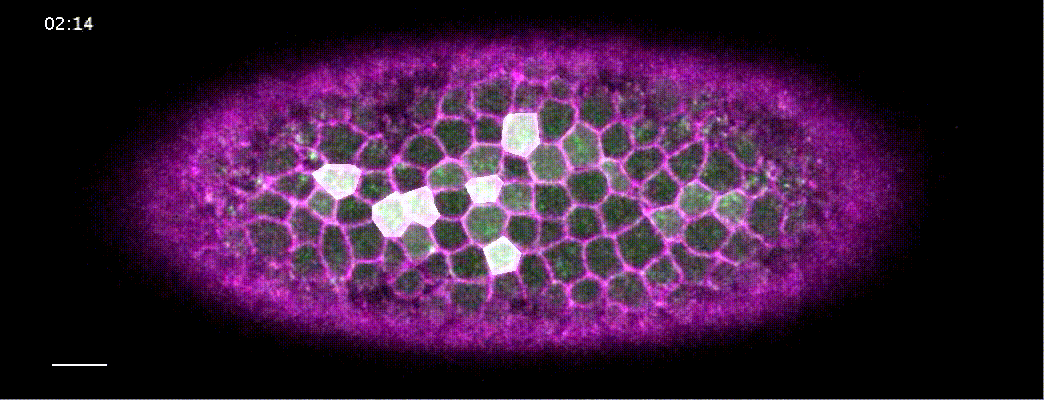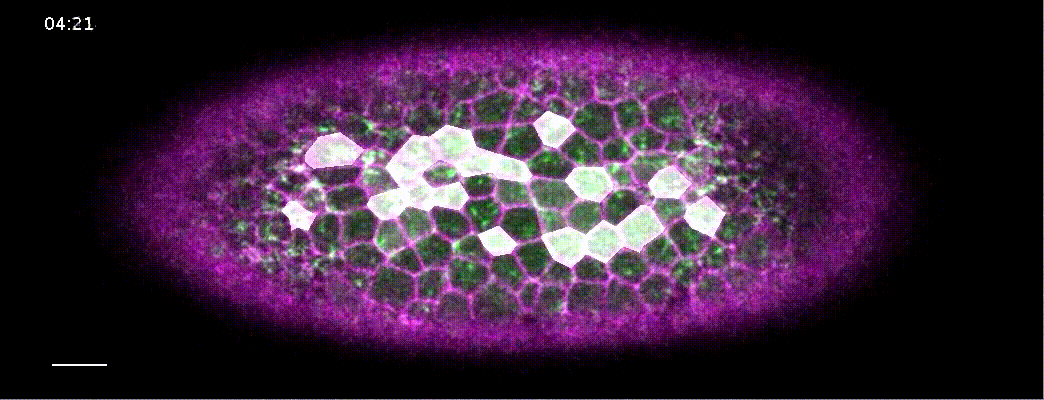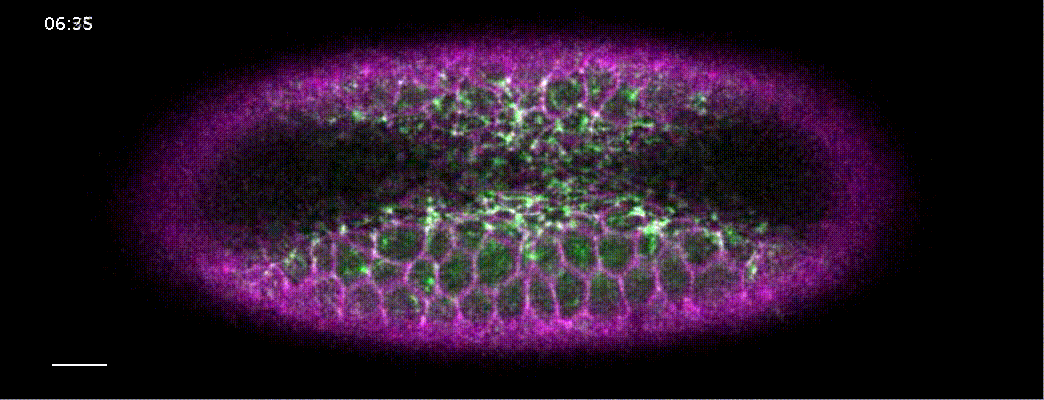
How does a contracting epithelium tolerate heterogeneity in cell size?
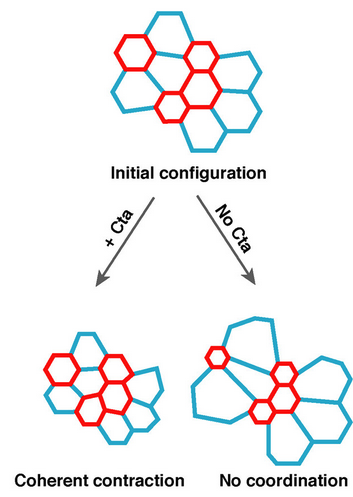
Using my computational method, I analyzed a class of developmental mutants who fail to mount ‘coherent’ contractions. In these mutants, a subset of cells in the mesoderm successfully constrict their apical surface, while the rest of the cells end up with expanded apices, resulting in failed gastrulation.
These mutations encode genes in the Drosophila Gα12/13 pathway, including the gene concertina (cta), which activates RhoA GTPase to control actin-myosin dynamics.
I found that, contrary to previous models that suggested these pathways propagated a contraction signal throughout the tissue, these pathways actually acted cell-autonomously. They ensured that the apical cortex in each contracting cell is robustly organized to withstand heterogeneity in apical sizes. In cta mutants, cells that are initially larger than their neighbors cannot sustain enough tension throughout its apical cortext, and will be pulled apart by its contracting neighbors.
Thus, this pathway buffers the contracting mesoderm against heterogeneity in cell size and ensures robust morphogenesis. (Xie et al., 2016)